Abstract
Introduction
Aplastic anemia (AA) is characterized by a hypoplastic marrow and bone marrow failure (BMF), leading to peripheral pancytopenia. The treatment for AA currently consists of either hematopoietic stem cell transplant (HSCT) or immunosuppressive therapy (IST) with anti-thymocyte globulin and cyclosporine. We have previously reported poor responses to IST in Indian children with AA while adults (>15 years) show responses of 60%. Therefore, we wanted to study if we could identify constitutional variants in children and young adults with AA.
Methods
We included 102 young patients (≤40 years of age) diagnosed to have AA between year Jan 2006 to April 2021. Genomic DNA was extracted from peripheral blood and relative telomere length (rTL) was measured using quantitative real-time PCR (qPCR). The DNA was used to perform targeted gene capture using a custom capture kit which covered 2196 genes associated with various hematological disorders. Among the 2196 genes, the analysis was restricted to 96 genes associated with AA/IBMFS. Bioinformatics analysis was carried out using Genome Analysis ToolKit (GATK) best practices pipeline. Based on the American College of Medical Genetics and Genomics (ACMG) guidelines, the variants were labelled as pathogenic/likely pathogenic/variant of uncertain significance (VUS)/likely benign/benign. The candidate variants were validated by Sanger sequencing.
Results
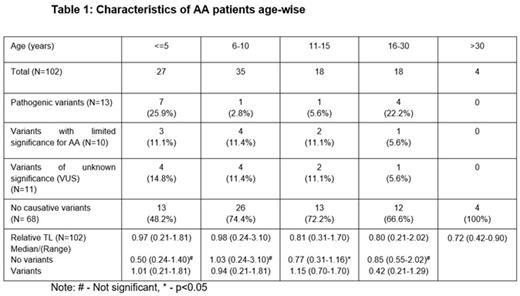
The median age of patients was 9 (0-40) years, including 56 males (55%) and 46 females (45%). There were 15 patients with non-severe AA (NSAA) (15%), 63 with severe AA (SAA) (62%) and 24 with very severe AA (VSAA) (23%). The median relative telomere length of the entire cohort was 0.85 (0.21-3.10). The characteristics of patients age-wise are mentioned in Table 1. Genetic variants were identified in 34 patients (33.3%). This included germline pathological (PAT) genetic variants in 12.7%, variants of limited significance in 9.8% and variants of uncertain significance (VUS) in 10.7% patients. Of the 13 patients who had PAT variants, 6 patients had variants in telomere associated genes. This includes 2 patients with splice site and missense mutation in TINF2 (c.1221+5G>A & R282H); 2 patients with missense mutation in TERT (C828W & S947C); and 2 patients with frameshift and nonsense mutation in RTEL1 (L962S & R1010X). Homozygous MPL mutations [L79Q, R522T & P530L(n=3)] were observed in 5 patients. Two patients had PAT variants in DNAJC21 and NLRP12. Eleven VUS variants were present in the following genes, ATM (n=3), TINF2 (n=1), WRAP53 (n=1), BRCA2 (n=1), AK2 (n=1), FANCA (n=1), FANCN (n=1), ERCC6L2 (n=1) & PRF1 (n=1). The incidence of mutations in the age group ≤5, 6-10, 11-15 and 16-30 years were 51.8%, 25.6%, 27.8% & 33.4% respectively. There was significant difference in median rTL between patients with variants and no variants in the age-group 11-15 years (p=0.043).
Discussion
Genetic analyses studies in aplastic anemia patients were confined to single genes or limited gene sets. Keel et al., reported 5.1% of AA patients carried pathogenic variants in inherited BMF/MDS genes. We observed a high frequency of causative genetic variants (37%) in children (<10 years). Our study results highlight the importance of recognizing pathogenic and VUS genetic variants contributing to the pathogenesis of AA in the lower age group.
Mathews: Christian Medical College: Patents & Royalties: US 2020/0345770 A1 - Pub.Date Nov.5, 2020; AML: Other: Co-Inventor.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal